Protein haze formation in wines
Hazy wines, especially whites, are perceived as faulty by most consumers, so that securing wine stability prior to bottling is an essential step of the winemaking process. Protein instability leading to haze in wines is one of the most important instabilities that winemakers face, particularly for white, rosé and sparkling wine production.1 If not removed during winemaking, grape proteins will be found in the bottled wines in a soluble state, in which case the wine is seen as limpid. During storage and transportation these proteins can slowly denature thus becoming insoluble and aggregate with each other. As these aggregates become larger they scatter more light and we see these particles as haze (Figure 1).

Not all proteins found in wine are responsible for wine hazing. The unstable proteins are only those that the plant produces during ripening as a way to defend itself against pathogens. Because of these characteristics they are named pathogenesis-related (PR) proteins, among which the two major sub-classes in wine are thaumatin-like proteins (TLPs) and chitinases. PR proteins are globular proteins are resistant to proteases and to low pHs, and therefore they ‘survive’ the winemaking process better than other proteins. This robustness is the issue, because if they were more fragile they would precipitate during winemaking and not after bottling to cause hazes.
In recent years our knowledge about the haze forming proteins improved significantly.2 We can now understand and explain accurately the role of many wine constituents including single proteins, polysaccharides, phenolics, ionic strength, sulphur dioxide, organic acids and sulfate on grape protein aggregation.2 Another recent key finding was that the temperature plays a key role in the onset of protein aggregation, and differences in the unfolding temperature of chitinases (55 °C) and TLPs (62 °C) were detected.3 Moreover, once heated chitinases stay unfolded upon cooling (irreversible unfolding), while TLPs refold (reversible unfolding). Chitinases were also more reactive with other wine macromolecules than TLPs, formed bigger aggregates and were more prone to precipitate.4 Therefore chitinases is considered as the major player in heat-induced haze in wines, even if other experiments proved that some TLPs isoform can form haze as well.5
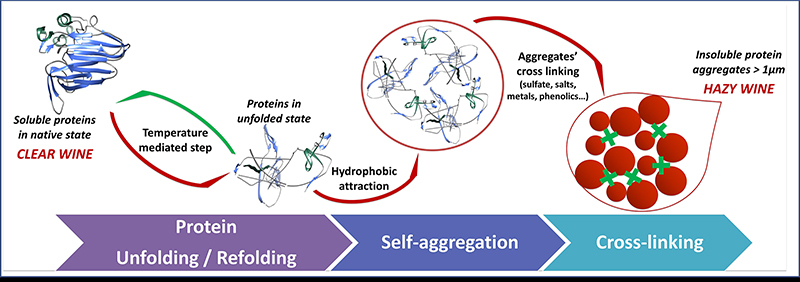
A general scheme showing the ‘life cycle’ of proteins in wine is summarised in Figure 2. Proteins in soluble state are found in their folded structure (Figure 2, on the left). However, this folded structure can be lost (unfolding), a step that is commonly mediated by inappropriate storage temperatures. Once the protein is in the wine in an unfolded structure, it exposes its hydrophobic binding sites and thus protein self-aggregation is induced via hydrophobic attractions with other unfolded proteins. With time, the aggregates can grow and cross-link with other aggregates until they reach a size that makes them visible to the naked eye (1 µm).
[Enlarged image]
Nowadays the chances of finding a hazy wine in a bottle shop are quite limited. This is the result of the prevention strategy used in commercial winemaking, which for several decades has been using bentonite, a clay cation exchanger able to bind the grape proteins. Bentonite is used worldwide as it is a treatment very effective in removing the grape proteins responsible for haze formation. However, its application has several drawbacks as its use tends to tie up tank time, causes volume and quality loss and presents waste disposal challenges.1 Quality degradation and loss of wine through bentonite usage has been estimated to cost the global wine industry around US$ 1 billion per year.6 Consequently, winemakers aim to use the minimum amount of bentonite required for protein stability and would welcome the introduction of alternatives with fewer drawbacks than the current practice. This need has driven research in the field, and an overview of the main findings to date will be given in the next section.
Alternative techniques for wine protein stabilization
Given that the mechanisms of wine protein haze formation is now well defined, we are well equipped to explore targeted strategies alternative to bentonite for preventing wine haze. Two in particular have been/are being investigated with different levels of success. These are relying on: i) the use of proteolytic enzymes to degrade the grape and wine proteins; ii) and the use of novel fining agents alternative to bentonite to remove proteins.
Protein degradation using enzymes
Degrading haze-forming proteins in wine with enzymes is the most attractive option as it would minimize wine volume loss and aroma stripping. Ideally, effective enzymes would be added to grape juice or ferment without the need for later removal, similarly to common practices as pectinases and glucanases additions.7 The degradation products of grape proteins may also be utilized by yeast as nitrogen sources, potentially reducing the frequent need for nitrogen additions (as diammonium phosphate) and improving wine aroma quality.8
To date, the search for enzymes able to degrade haze-forming proteins has focused on proteases that could degrade proteins under winemaking conditions. This proved to be a particularly challenging task as the selected enzymes would need to work at low temperatures typical of winemaking, to be active at wine pH and to break down proteins (chitinases and TLPs) very resistant to proteases.
A promising new strategy was recently developed.9 It consisted of adding an enzyme tolerant to acidic pH and putting it to work on the proteins after they have unfolded, which is a time when they are much more susceptible to enzyme attack. The selected protease (named AGP), when added to clarified grape juice and combined with short-term heating (75 °C for 1 min), has shown excellent results in removing haze-causing proteins (80-90% total protein reduction) and resulted in wines that were heat stable and almost completely free from haze-forming proteins. Chemical and sensory results indicated that there were no significant changes to the main physicochemical parameters and or wine preference.9 This combination treatment of protease addition with flash pasteurization has been shown to be effective at industrial scale, and its use has recently been approved for Australian and New Zealand winemaking. Given that the cost of this approach compared favorably to bentonite treatment,9 this solution is the only potentially cost-effective and commercially-viable bentonite alternative.
Other proteases are also currently being investigated that are active at winemaking temperatures and are specific against grape haze-forming proteins. Recent investigations have focused proteases from Botrytis cinerea, as grapes infected by Botrytis were found to have significantly lower concentrations of PR-proteins than juice from healthy grapes.10,11 One particular protease from Botrytis cinerea, BcAP8, has proven to be effective against grape chitinases during juice fermentation without the need for heating.12 When BcAP8 was added to juice prior to fermentation, the resulting wines produced significantly less heat-induced protein haze than wines made without BcAP8.
«Another enzyme that has been recently researched is stem bromelain, a proteases from pineapple that has shown some potential to reduce white wine protein haze when used both in free or immobilised form on a chitosan support.»
Another enzyme that has been recently researched is stem bromelain, a proteases from pineapple that has shown some potential to reduce white wine protein haze when used both in free or immobilised form on a chitosan support,13 even if several steps will be needed to make it a commercially viable option including the reduction of the amount of enzyme required and tests on pilot scale rather than laboratory scales.
Other potential sources of proteases that are active at wine pH include endogenous winemaking sources such as grapes, yeasts, and bacteria.14-16 Acid-tolerant yeasts and spoilage microbes are known to secrete proteases at wine pH, although the secreted protease activity is generally not sufficient to stabilize the wines.17,18 An extracellular pepsin-like aspartic acid protease of 72 kDa was characterized from a Saccharomyces cerevisiae isolate,19 one of the few isolates that secrete protease activity. The secreted yeast protease activity discovered by Younes et al.19,20 was active at wine pH during grape juice fermentation, although it did not affect grape PR-proteins until after fermentation when the wine was incubated at 38 °C for prolonged periods. The yeast strain that secretes protease activity is not currently used commercially in winemaking, although it could be used as a tool to develop new industrial wine yeast strains that secrete protease.
Very recently, the activity of an aspartic protease (MpAPr1) secreted by the wine yeast Metschnikowia pulcherrima was characterised and described.21 Similarly to the proteases from Saccharomyces cerevisiae, it is of interest that another wine yeast has shown the ability to secrete a proteases that was shown to have some activity against haze-forming grape proteins, however further research is being carried out to attempt to make this a viable option in the future.
Protein removal using novel fining agents
Many novel fining agents and other protein removal techniques with the potential to replace bentonite have been explored in recent years. These include carrageenan, chitin, zirconium dioxide, packed-bed cation exchangers, as well as magnetic nanoparticles.2 Obviously, being the mechanism of action of novel fining agents similar to that of bentonite, they must meet several criteria to effectively compete with bentonite. In particular, they must be cost effective, non-toxic, minimize wine losses and must not degrade wine quality, at least not more than bentonite does.
Carrageenan, a negatively-charged polysaccharides extracted from seaweeds, showed the potential to become a bentonite alternative.22 Carrageenan is a food grade polysaccharide already used for protein stabilization in the beer industry. It has been shown to be effective in stabilizing white wines at low addition rates (125-250 mg/L), using only one third or less than the bentonite concentration required.23 Carrageenan has been found to produce no deleterious sensory impacts compared to bentonite treated wines.24 While those results are promising, residual amounts of carrageenan in treated wines can potentially induce haze formation,22,23 and this is likely to restrict commercial viability. Further studies are currently being undertaken to solve the issues highlighted by previous studies and this will likely result in a product that could become commercially viable in the near future.
Another protein adsorbing polysaccharide studied is chitin. Chitin is a component of crustacean exoskeletons and, alongside its derivatives, such as chitosan, is widely used in industrial processes, including as a thickening agent for processed foods and in pharmaceuticals.25 The structure of chitin also makes it selective for binding to chitinases and in-line systems containing chitin can be effective in removing these particular PR-proteins from wine.26 Chitin could potentially have serious sensory impacts, however, by removing favourable wine components such as positive aroma compounds.27
«The ultimate objective of novel fining agents and protein-adsorbing materials for the wine industry is to achieve wine stabilization using in-line applications with minimum wine loss and no extra processing steps.»
The ultimate objective of novel fining agents and protein-adsorbing materials for the wine industry is to achieve wine stabilization using in-line applications with minimum wine loss and no extra processing steps. Zirconium dioxide (ZrO2) is a readily available protein-adsorbing material that can be regenerated and thus reused.28 ZrO2 has shown the potential to remove haze-forming proteins when tested in continuous and batch-wise application both during or post fermentation.29,30 However, despite ZrO2 showing promise and the ability to be regenerated with a simple washing procedure,31 issues with the flow rates and high dosages required currently limit its commercial viability.
A very recent study proposes the use of acrylic acid plasma-coated magnetic nanoparticles as a tool for the selective removal of haze-forming pathogenesis-related proteins from wine.32 This study looked at nine white wines and showed that this new separation method was very effective in removing both pathogenesis-related protein classes, TLPs and chitinases from white wines even when the wine contains high amount of proteins. Additionally, the treatment did not alter the phenolic composition of the wines and therefore has the potential to become a viable alternative to bentonite in the future.
Conclusions
The improved understanding of the mechanisms of haze formation that we now have has allowed and hopefully will continue allowing for the faster development of more targeted techniques to prevent protein hazes forming in bottled white wines.
Several solutions have been investigated, with one currently being viable commercially in some countries (flash pasteurization combined with proteases),9 and several being very promising and likely to become commercially viable in the future such as carrageenan,23 magnetic nanoparticles,32 and possible some proteases secreted from wine yeasts.21 The ideal and preferred solution would certainly be the finding of a proteolytic enzyme active at normal winemaking conditions, as this will ultimately benefit wine makers worldwide, but despite the continuous effort made by research groups is several countries, it seems very likely that it will take still some time for this solution to become reality.
References
1. Waters EJ. Alexander G. Muhlack R, Pocock KF, Colby C, O’Neill BK, Høj, PB, Jones P: Preventing protein haze in bottled white wine. Aust J Grape Wine Res 2005; 11 (2): 215-25.
2. Van Sluyter SC, McRae JM, Falconer RJ, Smith PA, Bacic A, Waters EJ, Marangon M: Wine protein haze: mechanisms of formation and advances in prevention. J Agric Food Chem 2015; 63 (16): 4020-30.
3. Falconer RJ, Marangon M, Van Sluyter SC, Neilson KA, Chan C, Waters EJ: Thermal stability of thaumatin-like protein, chitinase, and invertase isolated from Sauvignon blanc and Semillon juice and their role in haze formation in wine. J Agric Food Chem 2010; 58 (2): 975-80.
4. Marangon M, Van Sluyter SC, Neilson KA, Chan C, Haynes PA, Waters EJ, Falconer RJ: Roles of grape thaumatin-like protein and chitinase in white wine haze formation. J Agric Food Chem 2011; 59 (2): 733-40.
5. Marangon M, Van Sluyter SC, Waters EJ, Menz RI: Structure of Haze Forming Proteins in White Wines: Vitis vinifera Thaumatin-Like Proteins. PLoS One 2014; 9 (12): e113757.
6. Majewski P, Barbalet A, Waters EJ: $1 billion hidden cost of bentonite fining. Aust New Zeal Grapegrow Winemak 2011; No. 569: 58-62.
7. Moreno-Arribas MV, Polo MC: Winemaking Biochemistry and Microbiology: Current Knowledge and Future Trends. Crit Rev Food Sci Nutr 2005; 45 (4): 265-86.
8. Pretorius IS: Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast 2000; 16 (8): 675-729.
9. Marangon M, Van Sluyter SC, Robinson EMC, Muhlack RA, Holt HE, Haynes PA, Godden PW, Smith PA, Waters EJ: Degradation of white wine haze proteins by Aspergillopepsin I and II during juice flash pasteurization. Food Chem 2012; 135 (3): 1157-65.
10. Girbau T, Stummer BE, Pocock KF, Baldock GA, Scott ES, Waters EJ: The effect of Uncinula necator (powdery mildew) and Botrytis cinerea infection of grapes on the levels of haze-forming pathogenesis-related proteins in grape juice and wine. Aust J Grape Wine Res 2004; 10 (2): 125-33.
11. Marchal R, Warchol M, Cilindre C, Jeandet P.:Evidence for protein degradation by Botrytis cinerea and relationships with alteration of synthetic wine foaming properties. J Agric Food Chem 2006; 54 (14): 5157-65.
12. Van Sluyter SC, Warnock NI, Schmidt S, Anderson P, van Kan JAL, Bacic A, Waters EJ: Aspartic acid protease from Botrytis cinerea removes haze-forming proteins during white winemaking. J Agric Food Chem 2013; 61 (40): 9705-11.
13. Benucci I, Esti M, Liburdi K: Effect of free and immobilised stem bromelain on protein haze in white wine. Aust J Grape Wine Res 2014; 20 (3): 347-52.
14. Monteiro S, Piçarra-Pereira M, Mesquita PR, Loureiro VB, Teixeira A, Ferreira RB: The wide diversity of structurally similar wine proteins. J Agric Food Chem 2001; 49 (8): 3999-4010.
15. Manteau S, Lambert B, Jeandet P, Legendre L: Changes in Chitinase and Thaumatin-like Pathogenesis-Related Proteins of Grape Berries during the Champagne Winemaking Process. Am J Enol Vitic 2003, 4 (April): 267-72.
16. Waters EJ, Hayasaka Y, Tattersall DB, Adams KS, Williams PJ: Sequence Analysis of Grape (Vitis vinifera) Berry Chitinases That Cause Haze Formation in Wines. J Agric Food Chem 1998; 46 (12): 4950-7.
17. Dizy M, Bisson LF, Endowed MAA, Avenue OS: Proteolytic activity of yeast strains during grape juice fermentation. Am J Enol Vitic 2000; 51 (2): 155-67.
18. Strauss MLA, Jolly NP, Lambrechts MG, van Rensburg P: Screening for the production of extracellular hydrolytic enzymes by non-Saccharomyces wine yeasts. J Appl Microbiol 2001; 91 (1): 182-90.
19. Younes B, Cilindre C, Villaume S, Parmentier M, Jeandet P, Vasserot Y: Evidence for an extracellular acid proteolytic activity secreted by living cells of Saccharomyces cerevisiae PlR1: impact on grape proteins. J Agric Food Chem 2011; 59 (11): 6239-46.
20. Younes B, Cilindre C, Jeandet P, Vasserot Y: Enzymatic hydrolysis of thermo-sensitive grape proteins by a yeast protease as revealed by a proteomic approach. Food Res Int 2013; 54 (1): 1298-301.
21. Theron LW, Bely M, Divol B: Characterisation of the enzymatic properties of MpAPr1, an aspartic protease secreted by the wine yeast Metschnikowia pulcherrima. J Sci Food Agric 2017; 97 (11): 3584-93.
22. Cabello-Pasini A, Victoria-Cota N, Macias-Carranza V, Hernandez-Garibay E, Muñiz-Salazar R: Clarification of wines using polysaccharides extracted from seaweeds. Am J Enol Vitic 2005; 56 (1): 52-9.
23. Marangon M, Stockdale VJ, Munro P, Trethewey T, Schulkin A, Holt HE, Smith PA: Addition of carrageenan at different stages of winemaking for white wine protein stabilization. J Agric Food Chem 2013; 61 (26): 6516-24.
24. Marangon M, Lucchetta M, Duan D, Stockdale VJ, Hart A, Rogers PJ, Waters EJ: Protein removal from a Chardonnay juice by addition of carrageenan and pectin. Aust J Grape Wine Res 2012; 18 (2): 194-202.
25. Shahidi F, Arachchi JKV, Jeon Y-J: Food applications of chitin and chitosans. Trends Food Sci Technol 1999; 10 (2): 37-51.
26. Vincenzi S, Polesani M, Curioni A: Removal of specific protein components by chitin enhances protein stability in a white wine. Am J Enol Vitic 2005; 56: 246-54.
27. Spagna G, Pifferi PG, Rangoni C, Mattivi F, Nicolini G, Palmonari R: The stabilization of white wines by adsorption of phenolic compounds on chitin and chitosan. Food Res. Int 1996; 29 (3-4): 241-8.
28. Pashova V, Güell C, López F: White wine continuous protein stabilization by packed column. J Agric Food Chem 2004; 52 (6): 1558-63.
29. Marangon M, Lucchetta M, Waters EJ: Protein stabilisation of white wines using zirconium dioxide enclosed in a metallic cage. Aust J Grape Wine Res 2011; 17 (1): 28-35.
30. Lucchetta M, Pocock KF, Waters EJ, Marangon M: Use of zirconium dioxide during fermentation as an alternative to protein fining with bentonite for white wines. Am J Enol Vitic 2013; 64 (3): 400-4.
31. Marangon M, Lucchetta M, Waters EJ: Protein stabilisation of white wines using zirconium dioxide enclosed in a metallic cage. Aust J Grape Wine Res 2011; 17 (1): 28-35.
32. Mierczynska-Vasilev A, Boyer P, Vasilev K, Smith PA: A novel technology for the rapid, selective, magnetic removal of pathogenesis-related proteins from wines. Food Chem 2017; 232: 508-14.